Promising clinical results of immune nurturing ingredient reducing the risk of respiratory and gastrointestinal infections in infancy.
A review of the most recent evidence demonstrating the clinical benefits of infant formula containing human milk oligosaccharides (HMOs).**
Cow’s milk protein allergy (CMPA) is an immune-mediated disease characterised by allergic reactions to cow’s milk protein. CMPA is also associated with increased gut permeability and an altered gut microbiota which affects the maturation of the immune system, leaving infants at a higher risk of developing infections and future allergies.1-3
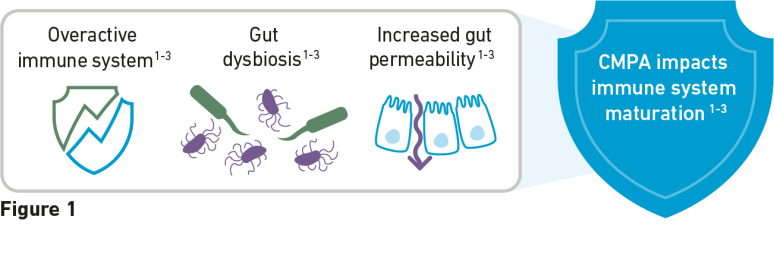

Children diagnosed with CMPA in infancy are at a higher risk of respiratory atopy and atopic dermatitis by 10 years of age.2

Clinical evidence has demonstrated that Human Milk Oligosaccharides (HMOs) in breast milk can nurture an infant’s immune system and reduce the risk of infections.4-7
HMOs IN BREAST MILK SUPPORT THE IMMUNE SYSTEM
Breast milk is the gold standard for infant nutrition including those with CMPA.8 Breast milk contains large quantities of HMOs, non-nutritive components known to support infants’ developing immune systems.4 They represent the third most abundant solid component after lactose and lipids. Out of approximately 200 HMOs, 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT) are two of the most significant, typically accounting for more than 30%.4
HMOs are identified as the most important bifidogenic factor in human milk.9,10 Beyond their role as prebiotics, HMOs in breast milk have significant impact on the microbiota. They provide protection against certain harmful pathogens in the body and mostly via the gut due to their antimicrobial and adhesive properties, can prevent growth and proliferation of harmful bacteria by modifying the host microbe interaction.11,12
HMOs** ADDED TO SPECIALISED INFANT FORMULA MAY SUPPORT THE IMMUNE SYSTEM AND PROMOTE THE DEVELOPMENT OF MICROBIOTA IN THE GUT
For more than 50 years, HMOs, and more specifically 2-’FL and LNnT, have been an exciting area of research at Nestlé. This has led to a number of clinical trials with infant formula products supplemented with two structurally identical HMOs (2’-FL and LNnT) to those found in breast milk, in both healthy infants13 and infants with CMPA.14-17
- Research shows that inclusion of these two HMOs (2’-FL and LNnT) that are structurally identical to those found in breast milk, supports safe growth in formula-fed babies and that they are well tolerated.13,14,15,17
- Studies (in formula-fed infants with and without CMPA) using infant formula containing these two HMOs (2’-FL and LNnT) structurally identical to those found in breast milk, show growing evidence for:
- Selective growth of beneficial gut microbiota*13,14,16
- Reduction in infections*13,15,16
- A reduction in antibiotics and antipyretic medication over 12 months.*13,15,16
CINNAMON STUDY (2022)
Infants with Cow’s Milk Protein Allergy (CMPA) provided an extensively hydrolysed infant formula supplemented with HMOs (2’-FL and LNnT)** showed15:
- Normal growth from enrolment to 4 month follow up
- A protective effect against respiratory and ear infections in the first year of life*
- A nonsignificant reduction of up to 11% in the use of antibiotic and antipyretic medication use from enrolment to 12 months follow up
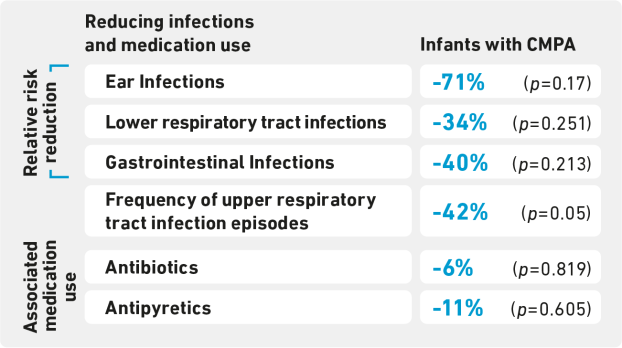
n=190; Age: 3.2-12 months; mean trial duration: 8.8 months. 41 Clinical sites in Europe and 3 sites in Singapore. Growth parameters were studied over a 4-month period (primary study endpoint). This study was sponsored by Nestlé Health Science. Test formula was an extensively hydrolysed formula supplemented with two HMOs; 2’-FL and LNnT**
PLATYPUS STUDY
Infants with Cow’s Milk Protein Allergy (CMPA) provided an amino-acid based infant formula Alfamino® containing two HMOs (2’-FL and LNnt)** showed:14
- Adequate growth and the infant formula was well tolerated with an excellent safety profile
- A significant early enrichment of genus Bifidobacterium (including HMO-utilising bifidobacteria) in the gut (Figure 2 below).*
- Relative abundance (HMOs-utilising bifidobacteria)* from:
- 0-1 months (V0 – V1) (p=0.07)
- 4 months (V4) (p=0.017)
- 12 months (V5) (p=0.005)
- ↓ in abundance of Proteobacteria after 12 months* (V5); a marker phylum of gut dysbiosis (p=0.017)
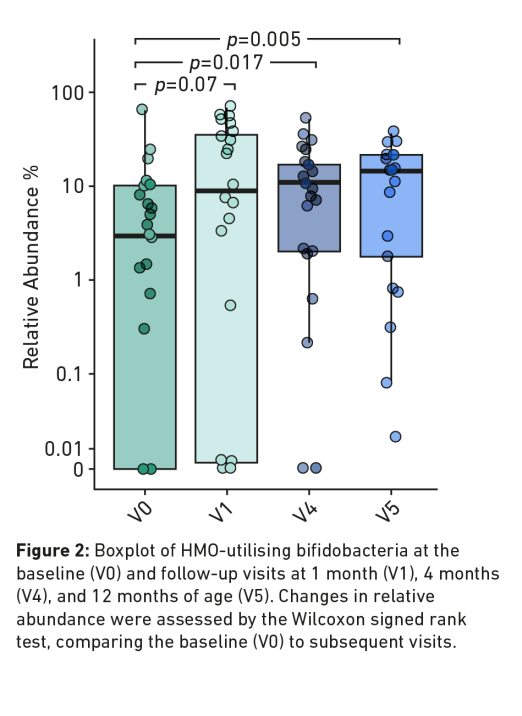
This open-label, non-randomised, multicentre trial was conducted in six clinical sites in Australia between December 2018 and March 2021 using ALFAMINO® containing two HMOs: 2′-FL and LNnT. 32 Term infants aged 1-8 months with physician-diagnosed moderate-to-severe CMPA were enrolled. Growth parameters were studied over a 4-month period (primary study endpoint). Infants were offered to remain on the study formula until 12 months of age (secondary study endpoint). This study was sponsored by Nestlé Health Science. At enrolment, 50.0% of infants were being fed an extensively hydrolysed formula, 9.4% infants received a hydrolysed rice formula and 40.6% received an amino acid-based formula without HMO.
*Reported as secondary outcomes.
**Structurally identical to those found in breast milk.
Further research will help confirm these promising clinical results and explore the mechanisms by which HMOs provide immune support and modulation, reduce the risk of respiratory and gastrointestinal infections in infancy and establish the clinical effects associated with HMO-induced microbiome changes.
Request samples of PBS-listed ALFAMINO® for professional evaluation. Click here. ALFAMINO® is the FIRST AND ONLY amino acid-based formula in Australia with two Human Milk Oligosaccharides (HMOs) – 2’-FL and LNnT, that are structurally identical to those found in breast milk.
For more information call 1800 671 628 or visit Alfamino® | Infant Food Allergy | Nestle Health Science or request to meet a Nestle Health representative here.
INFORMATION FOR HEALTHCARE PROFESSIONAL USE ONLY.
BREASTFEEDING IS BEST FOR BABIES. ALFAMINO® is an infant formula product for special dietary use and must be used under medical supervision. It is not suitable for general use.
IMPORTANT NOTICE: Breast milk is best for babies and provides ideal nutrition. Good maternal nutrition is important for the preparation and maintenance of breastfeeding. Introducing partial bottle feeding could negatively affect breastfeeding and reversing a decision not to breastfeed is difficult. Professional advice should be followed on infant feeding. Infant formula should be prepared and used exactly as directed or it could pose a health hazard. The preparation requirements and weekly cost of providing infant formula until 12 months of age should be considered before making a decision to formula feed. Mothers should be encouraged to continue breastfeeding even when their infants have cow’s milk protein allergy. If a decision to use an infant formula for special dietary use is taken, it must be used under medical supervision.
References:
- Juntti H, et al. Acta Otolaryngol 1999;119(8):867-73.
- Tikkanen S, et al. Acta Paediatr 2000; 89(10):1174-80.
- Woicka-Kolejwa K, et al. Postepy Dermatol Alergol 2016;33(2):109-13
- Donovan SM, Comstock SS. Ann Nutr Metab 2016;69(suppl2):42-51.
- Garrido D, et al. Microbiology 2013;159(Pt 4):649-64.
- Ruiz-Palacious GM, et al. J Biol Chem 2003;278(16):14112-20.
- Bode L. Glycobiology 2012;22(9):1147–62
- Koletzko S, et al. J Pediatr Gastroenterol Nutr 2012;55(2):221-9.
- Lewis, Z.T et al. Microbiome. 2015;3:13.
- Sprenger, N. et al. PLoS ONE 2017;12:e0171814
- Santos A et al. EMJ Allergy Immunol. 2020;5[Suppl 2]:2-10.
- Moossavi S et al. Front. Pediatr. 2018;6:197
- Puccio G, et al. J. Pediatr Gastroenterol Nutr 2017;64(4):624-31.
- Gold et al. Nutrients 2022 May 30;14(11):229. (PLATYPUS)
- Vandenpals et al. Nutrients. 2022 Jan 26;14(3):530 (CINNAMON)
- Berger, B. et al. mBio. 2020 Mar 17;11(2):e03196-19.
- Nowak-Wegrzyn A, et al. Nutrients 2019;11(7):E1447
Sponsored by Nestlé Health Science.


